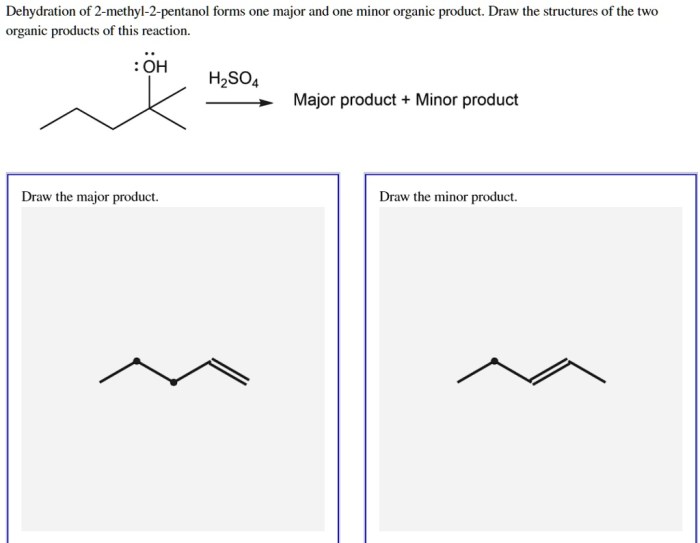
Draw the major product for the dehydration of 2 pentanol – The dehydration of 2-pentanol is a captivating topic in organic chemistry, offering insights into the intricate mechanisms and fascinating outcomes of this fundamental reaction. This comprehensive guide delves into the details of the dehydration process, exploring the formation of the major product and the factors that influence its regio- and stereoselectivity.
Our journey begins with an overview of dehydration reactions, highlighting their significance in organic synthesis. We then focus specifically on the dehydration of 2-pentanol, meticulously examining the reaction mechanism and the factors that govern the regio- and stereochemical outcomes.
Dehydration of 2-Pentanol
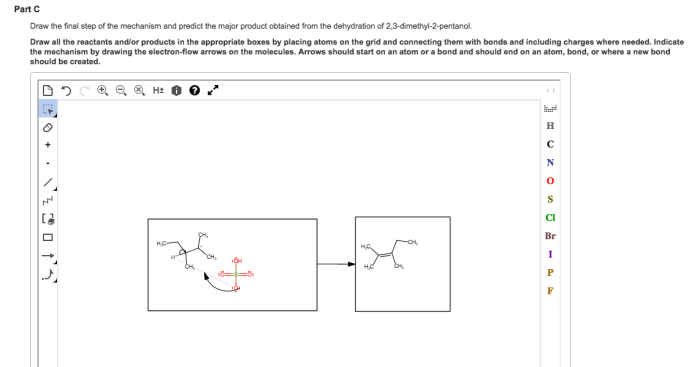
Dehydration reactions involve the removal of a water molecule from an organic compound, resulting in the formation of a double or triple bond. In the case of alcohols, dehydration leads to the formation of alkenes.
Dehydration of 2-Pentanol
The dehydration of 2-pentanol proceeds via an E2 elimination mechanism, which involves the simultaneous removal of a proton and a hydroxide ion from adjacent carbon atoms. This results in the formation of two products: 2-pentene and 1-pentene.
The major product of the reaction is 2-pentene, which is formed via the removal of a proton from the carbon atom adjacent to the hydroxyl group and a hydroxide ion from the hydroxyl group itself.
Regioselectivity
Regioselectivity in dehydration reactions refers to the preferential formation of one alkene isomer over the other. In the dehydration of 2-pentanol, the formation of 2-pentene is favored over 1-pentene due to the stability of the intermediate carbocation formed during the reaction.
Stereoselectivity
Stereoselectivity in dehydration reactions refers to the preferential formation of one stereoisomer over the other. In the dehydration of 2-pentanol, the major product, 2-pentene, is formed as a mixture of E and Z isomers. The E isomer is formed predominantly due to the steric hindrance caused by the methyl group on the carbon atom adjacent to the double bond.
Applications, Draw the major product for the dehydration of 2 pentanol
The dehydration of 2-pentanol has various applications in organic synthesis, including:
- The production of 2-pentene, which is used as a starting material for the synthesis of other chemicals, such as plastics and solvents.
- The removal of water from organic solvents.
- The dehydration of other alcohols to form alkenes.
Query Resolution: Draw The Major Product For The Dehydration Of 2 Pentanol
What is the major product of the dehydration of 2-pentanol?
The major product is 2-pentene, formed via the E2 elimination mechanism.
What factors influence the regioselectivity of the dehydration of 2-pentanol?
The regioselectivity is influenced by the stability of the carbocation intermediates, with the more substituted carbocation being more stable and leading to the major product.
What is the stereochemistry of the major product of the dehydration of 2-pentanol?
The major product is predominantly the E isomer due to the anti-periplanar orientation of the leaving group and the hydrogen being abstracted.